Despite its status as the shortest month of the year, February turned out to be quite eventful in events in the legislative sphere. Thus, the Ministry of Industry and Trade proposed to begin an experiment on labeling dietary supplements this year, and the Ministry of Health again extended the validity of accreditation certificates. In addition, Rospotrebnadzor commented on the situation with the return of medicines that arose with the abolition of the old Rules for the sale of certain types of goods. This and other things will be discussed in more detail in our traditional review of pharmaceutical legislation.
Returning drugs to a pharmacy
At the end of February, Rospotrebnadzor gave an answer to the main question that arose with the adoption of the new Government Resolution No. 2463 of December 31, 2020 - is it possible to return the medicine to the pharmacy?
Let us recall that in the previous Rules for the sale of certain types of goods, medicines were included in the list of goods that are not subject to exchange or return. In the new document, the list was renamed “list of goods not subject to exchange.” Thus, the question arose - it is impossible to exchange, but what about the return of medicines?
Rospotrebnadzor explained that the “return” of a product, according to Article 25 of the Law of 02/07/1992 No. 2300-1 “On the Protection of Consumer Rights”, is its “exchange” for money if a similar product is not available. And since drugs are included in the list of goods that cannot be exchanged, no return can be made.
How to file a claim
For the seller to return the purchase price, just follow simple instructions.
First of all, evidence is collected, and then you can begin to take action:
- Find a recipe, a cash receipt. If there is no receipt, find witnesses to the purchase.
- Compose a free-form application addressed to the head of the pharmacy, indicating your contact phone number, passport details, date, time of receipt, name of the product, reason for refusing the purchase and the desired result.
- Return to the pharmacy with the receipt, application and passport, hand over the documents to the pharmacist or pharmacist.
- Pick up the second copy of the claim.
It is better to attach copies of documents to the complaint. The originals will be useful for drawing up a statement of claim or contacting Rospotrebnadzor. Within 7 days from the receipt of the complaint, a pharmacy employee must contact the buyer and invite him to exchange medications or receive money.
Labeling of dietary supplements
At the very beginning of the month, the Ministry of Industry and Trade published a draft resolution on the start, from April 1, 2021, of an experiment on labeling biological active food additives with identification means, similar to medicines.
The experiment is planned to be carried out for 10 months - from April 1, 2021 to February 1, 2022. Let us remember that the pilot project on drug labeling took more than three years. At the same time, initially the experiment was supposed to end in 2021, but was later postponed to 2021.
For a detailed analysis of the draft resolution on the labeling of dietary supplements, as well as the market’s readiness for it, read our separate article.
Fines for marking
And continuing the topic of labeling - new fines for violating the rules of working with GIS MDLP, adopted by State Duma deputies in the first reading. The document proposes to add two new articles to the Code of Administrative Offenses - for the introduction and circulation of unlabeled drugs, as well as for failure to provide mandatory information about labeled goods to the system.
The punishment for such offenses will be a fine of 5 to 10 thousand rubles for officials and from 50 to 100 thousand rubles for legal entities.
Thus, in addition to the already existing Article 6.34 “Untimely entry of data into the system for monitoring the movement of medicinal products for medical use or entry of false data into it,” liability will be introduced for intentional failure to provide information to the labeling system, as well as for the circulation of drugs without labeling.
What to do?
Replace the drug. Apologize 10 times, and then immediately sound the alarm and find out how such a drug came to be sold. In this case, claims may come not only from buyers, but also from inspection authorities, and not only for the sale of a medicinal product, but also for the discovery of an expired drug on the sales floor (falls under a gross violation of licensing requirements). For gross violation of licensing requirements, the pharmacy faces administrative liability under Part 4 of Art. 14.1. Code of Administrative Offenses of the Russian Federation up to the suspension of activities (as an example, see Resolution of the Ninth Arbitration Court of Appeal dated January 26, 2011 N 09AP-32955/2010-AK).
The first candidates for inclusion in the VED list of 2022 have been named
In February, the first meeting of the Ministry of Health commission on the formation of drug lists finally took place. Based on its results, VED-2022 may include eight new positions:
| INN | Dosage form | Pharmacological group |
| 4-nitro-N-[(1RS)-1-(4-fluorophenyl)-2-(1-ethylpiperidin-4-yl)ethyl]benzamide hydrochloride | Concentrate for the preparation of solution for intravenous administration | Drugs for the treatment of heart disease |
| Gadoteric acid | Solution for intravenous administration | Contrast media |
| Delamanid | Film-coated tablets | Drugs active against mycobacteria |
| Bulevirtide | Lyophilisate for the preparation of solution for subcutaneous administration | Systemic antiviral drugs |
| Polymyxin B | Powder for solution for injection | Systemic antibacterial drugs |
| Alpelisib | Film-coated tablets | Antitumor drugs |
| Talazoparib | Film-coated tablets | Antitumor drugs |
| Pomalidomide | Capsules | Immunosuppressants |
In addition, the commission voted to exclude the drug “meldonium” in the dosage form “capsule” from the VED.
Let us remind you that the commission will hold its meetings several times throughout the year, so we can safely say that this list is not final. Follow the updates in our “News” section so as not to miss information about what other drugs may be added to or left the VED this year.
Renewal of accreditation
The Ministry of Health has once again extended the validity of accreditation certificates. All previously issued specialist certificates and accreditation certificates have been extended for 12 months if their validity expires before December 31, 2021. The same applies to all similar documents, the period of which has already been extended in accordance with Decree of the Government of the Russian Federation dated April 3, 2020 No. 440.
In addition, the Ministry of Health is suspending the procedure for obtaining specialist accreditation certificates until June 1. The exception is cases of undergoing primary accreditation after receiving higher or secondary medical education.
Work without accreditation
And continuing the topic of accreditation, the State Duma proposed to abandon the issuance of certificates on paper. This way, doctors and pharmacists will be able to start working immediately after completing the accreditation procedure, without waiting to receive a certificate form. Currently, employers cannot hire an employee who does not have a certificate, as this would be contrary to federal law. However, obtaining a certificate may take a long time, for example, due to the lack of necessary forms.
In this regard, amendments will be made to Article 69 of Law No. 323-FZ “On the Fundamentals of Health Protection”. Instead of a paper certificate, it will be possible to receive an extract from the Unified State System in the field of healthcare. The extract and paper license will have the same legal force.
What kind of money can you still get?
Starting from 2021, you can apply for a tax refund for any medicine. The list of drugs was canceled on June 17 by amendments to Federal Law No. 147-FZ.
A tax deduction is provided for all drugs that were prescribed by a doctor and purchased according to a prescription.
To return 13% of the amount spent, you must:
- collect prescriptions, payment receipts;
- fill out tax return 3-NDFL;
- submit documents to the tax office or submit them to the accounting department at your place of work.
If the declaration is completed correctly, money can be received in two ways:
- when calculating wages, the employer will take into account the amount of personal income tax to be deducted and will issue more money;
- After checking the provided data by the tax service, a transfer in the amount of 13% of the funds spent will be transferred to your bank account.
The tax deduction limit is 120 thousand rubles. You can receive partial compensation for any medications prescribed by the doctor to the applicant, spouse, or children. You can submit a declaration annually for the previous calendar year.
Extension of qualification categories
Another news on the topic of extending deadlines and moratoriums is that until January 1, 2022, certification of medical and pharmaceutical workers to obtain a qualification category will be suspended in Russia. This order of the Ministry of Health was approved in early February.
Simultaneously with the introduction of a moratorium on certification, the ministry is extending the validity of already assigned categories by 12 months if they expire between January 1 and December 31, 2021. This also applies to categories that were extended from February 1, 2021 to January 1, 2021 in accordance with Order No. 394n dated April 30, 2020.
Limitations for large networks
The State Duma adopted in the first reading a bill on restrictions on the number of pharmacies in large chains, as well as on maximum payments under marketing contracts. The document was submitted to the Duma back in March last year.
We published a full review of the document and experts’ opinions on the consequences of its final adoption in our separate article.
Let us briefly recall that the document will prohibit pharmacy chains from opening new outlets under their own brand if the chain’s turnover is more than 20% of the volume of all medicines and medical products sold in the city. In addition, first-principals will have the obligation to inform the buyer about the availability of the cheapest drugs within the framework of the INN of interest.
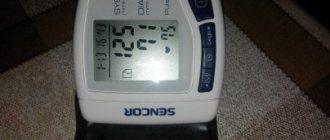
When will an examination help?
If the package is intact, and the appearance of the tablets and cough syrup corresponds to the description, an examination will help to identify hidden defects.
There are state laboratories in every city where they will conduct analysis and determine the compliance of medications with standards free of charge. If the suspicion that you have purchased a low-quality product turns out to be true, the conclusion of the quality control center can be brought to the pharmacy and you can get your money back.
When contacting the laboratory, you need to fill out a questionnaire, where in the appropriate columns indicate:
- name of the drug;
- what quantity was taken, for how long;
- what medications were taken in combination;
- the drug was purchased with a prescription or independently;
- what side effects appeared during use.
Along with the questionnaire, you must provide the medicine and packaging. It is better to clarify the required quantity by phone in advance.
Depending on the amount of active ingredient contained, you may be asked to bring 5-10 tablets, 20-100 ml of syrups, 4-6 ampoules. The packaging must indicate the expiration date and batch number. There is no need to submit receipts, prescriptions, or other documents to the laboratory.
On average, the examination takes 3-4 weeks. During this time, the manufacturer, upon request, will send a sample for comparison, and the laboratory will check the quality.
The test report will contain information about compliance with standards, the amount of active substance, but not about the effectiveness of the drug for a specific buyer.
You can submit tablets for research only before the expiration date.
Amendments to the rules of distance selling
The Ministry of Health presented a revised draft of amendments to the rules for the remote sale of medicines. The main previously announced amendment remained unchanged - to obtain permission to sell drugs, a pharmacy will no longer need to have 5 or more points of sale. Thus, “singles” will also be allowed to participate in distance trading.
According to the new draft amendments, pharmacies will be allowed to enter into contracts with several aggregators at the same time. In addition, for aggregators the requirement for the number of site visitors of at least 500 thousand people per year is excluded. To obtain permission from Roszdravnadzor to sell drugs online, an online aggregator will need to be registered as a legal entity for at least one year and have annual revenue of at least 5 billion rubles.
The latest change concerns drug delivery. The new version excludes mention of the possibility of delivering drugs to dispensing points. Thus, medications purchased remotely can only be delivered to the end consumer’s address.
Procedure
To return the purchased measuring device back to the pharmacy, you will need to draw up a document such as a claim. There are examples of how to write such papers on legal websites, but the applicant should also know some precise information regarding his case:
- The claim indicates the name of the institution (pharmacy) where the return of the tonometer is sent. You need to know the name of the pharmacy and the name of the manager, because this document is drawn up in his name.
- Next in the claim is the applicant’s data.
- The date, time of purchase, and other circumstances are indicated.
- The essence of the problem must be described accurately, reliably, without emotion.
- You should indicate the name of the product, its brand, model, configuration, serial number, and other data that will allow you to identify the device.
- The circumstances under which the marriage was discovered and how it manifested itself are described.
- It should be clarified that the defect was not visible during a visual inspection before paying for the purchase, it was only discovered at home.
- Demands are put forward to the seller - to return the money for the purchase, exchange it for another product, make repairs.
The claim is drawn up in two copies at once, one remains with the applicant, the second is sent to the pharmacy. Copies of the payment document for payment for the tonometer must be attached to the document, and information from it must be indicated in the text.
There are two ways to submit a claim. The first is to hand it over personally to the seller, the head of the pharmacy. But with a mandatory mark of delivery. The second is to send by registered mail with notification.
Effective date
On February 1, Part 1 of Article 3 of the Federal Law of July 31, 2020 No. 247-FZ “On Mandatory Requirements in the Russian Federation” came into force. According to it, any regulations (orders of ministries, government regulations or changes to federal laws) that in any way change mandatory requirements can only come into force on March 1 or September 1. But not earlier than 90 days after the day of official publication of the relevant regulatory legal act.
Thus, if the Ministry of Health suddenly decides to change the dispensing rules or the procedure for filling prescriptions, then all pharmacists will have at least 90 days, and at best six months, to prepare for the upcoming changes.
By the way, the law “On Mandatory Requirements” itself has many interesting points. This monumental document affects not only pharmaceutical activities, but also any areas of activity controlled by the state. Read our special article for a detailed analysis of the law.
Turnover of medical goods
Another serious problem in the medical goods market during the COVID-19 pandemic has been the turnover of in-demand products. Everyone probably remembers well how, after the publication of official recommendations from WHO and Rospotrebnadzor, medical masks and gloves practically disappeared from free sale in pharmacies and other organizations, and if they were sold, then at a price that was an order of magnitude higher than in the pre-coronavirus period. Under these conditions, the Government had no choice but to intervene in the situation, trying to streamline the activities of unscrupulous market participants.
For these purposes, Resolution No. 431 was adopted on April 3, 2021. It introduced a number of serious restrictions regarding priority goods, recorded in the annex to the regulatory document. These included gauze, medical masks and respirators, medical gloves and protective kits. The list of the most important innovations recorded by this resolution could include:
- restrictions on the size of the maximum markup on goods from the list, which at each stage of the trade chain leading from manufacturer to buyer should not exceed 10%;
- restrictions on the size of the maximum markup on these goods in the retail network, which should not exceed 10 kopecks per unit of goods.
To regulate the operation of the market, the resolution also introduced a special player - a regional operator, whose responsibilities included establishing efficiently operating channels for the supply of necessary goods in each subject of the Federation. The list of candidates for the role of such an operator could include companies that, at the time the resolution was adopted, had a valid wholesale pharmaceutical license.
In a word, this regulatory document has brought considerable confusion to this part of the market. However, its participants had not yet had time to properly adapt to the new rules, when just ten days later it was canceled. The relevant instructions were contained in Government Resolution No. 500 of April 13, 2021.
Export of medical goods
Despite the fact that in the context of the spread of the COVID-19 infection, international relations were frozen to some extent, this did not affect the issues of trade turnover in terms of medical goods. On the contrary, countries are actively helping each other with current developments in the fight against coronavirus, as well as in providing necessary goods to countries that need them - both through purchases at commercial prices and in the form of humanitarian assistance.
However, the Russian Government, having analyzed the situation in the initial phase of the pandemic, came to the conclusion that the active export of essential medical products could significantly aggravate their shortage in the domestic market. Therefore, in the first days of spring, Government Resolution No. 223 of March 2, 2021 was adopted, which temporarily banned the export of the most important products - masks, protective equipment, etc., making an exception for their export for the purpose of providing humanitarian assistance. Thanks to this, all the technological capabilities of Russian manufacturers were concentrated on the domestic market, which made it possible to overcome the resulting deficit in the shortest possible time.
After it was overcome, significant relaxations were made in this regard. First, Government Decree No. 413 of April 2, 2021 lifted the ban on transit shipments that began outside Russia and also ended outside its borders. In addition, the new resolution also did not apply to deliveries from one region of Russia to another if they required transportation through the territories of foreign states.
Two months after the adoption of the first resolution No. 223, a new regulatory document was issued - Government Resolution No. 637 of April 30, 2021. It lifted the restriction on the supply of in-demand goods to states that are members of the Eurasian Economic Union. And later, by decree No. 840 of June 9, 2021, it was finally allowed to export masks, gloves and other popular products to foreign countries. The only condition that still remains in force in this area is the need to monitor the situation to ensure that particularly large supplies do not cause damage to the domestic market. The implementation of such control based on an analysis of the parameters of the current state of affairs was entrusted to the Ministry of Industry and Trade.
At the moment, the procedure for regulating exports remains the same. However, even in these conditions, Russian manufacturers have already felt quite comfortable. According to the Ministry of Industry and Trade, the first contracts in this area have not only been concluded, but also completed, and in total about 20 Russian manufacturers have expressed their interest in working with potential foreign buyers. At the same time, this situation obviously did not cause a shortage of masks, gloves and protective suits in our country: however, this is justified - after all, today the capacities of Russian enterprises already allow the production of 11 million masks per day.
Register of mandatory requirements
And in addition to the implementation of the law “On Mandatory Requirements,” the Ministry of Economic Development, together with the Ministry of Digital Development, will form a special register of mandatory requirements, which will list all regulations that establish requirements for various types of activities.
It will be a database listing the mandatory requirements contained in various regulations: orders, regulations, sanitary rules, and so on. For each requirement, a link will be given to the corresponding document, the validity period of this requirement, a hyperlink to the checklist, as well as possible liability for non-compliance will be indicated. The register will be made publicly available to everyone.
The creation of the register was planned for March 1, from this date it should begin filling it with the necessary information.
Exchange and return of medicines by receipt
When purchasing goods or medical products, you must pick up a cash receipt. It will allow you to confirm the purchase of a specific item from the seller. The legislator provides for the buyer's right to refer to a witness if the payment document is lost.
The basis for a return is the fault of the seller’s actions, as well as the presence of various defects on the packaging and the product itself, which makes it a low-quality product.
Is it possible to return
Medicines belong to a special category of goods that require special storage conditions. Pharmacies guarantee compliance with the rules for handling them.
By transferring drugs to the patient, pharmacists lose the opportunity to guarantee the safety of tablets, mixtures, and so on. The law describes situations when a person can return medications.
Which ones can be returned?
Pharmacy organizations are obliged to accept back pharmaceutical products of inadequate quality:
- The medicine has expired on the date of purchase. It becomes dangerous to the life and health of the patient.
- There is no information on the packaging about production time or shelf life. This includes cases when the text is erased, taped up, the packaging is torn in the place of the description, if some numbers are indicated on the box, and others on the bottle or ampoule. The latter situation requires the seizure of an entire batch of medicines.
- After opening the package, it turned out that there was no information about the rules for using the purchased goods.
- A difference was found between the description of the tablets and the contents of the box.
- Chips, cracks, scratches on containers, allowing one to doubt the tightness. The same goes for cardboard boxes. Any defects indicate non-compliance with the conditions of transportation and storage.
Pharmacies are required to take back low-quality drugs.
If a medicine is sold without a prescription and requires a doctor's prescription, it can be replaced, but the quality does not matter.
Which ones can be exchanged?
Any identified deficiencies are irreparable; using purchased substances is dangerous. The patient has the right to demand elimination of violations. If the pharmacy has the required product, an exchange takes place, otherwise the money will be returned.